Doctors Co., Ltd., (head office: Minato-ku, Tokyo; President & CEO: Takao Yanagawa; hereinafter referred to as “DOCTORS”), which provides total comprehensive support from development to provision of a platform for digital healthcare services, hereby announces that it has launched the provision of Doctors Cloud® MD, a support service for obtaining the approval and certification of medical devices that meets the new needs of full-scale medical DX.
Doctors Cloud® MD is a new service that DOCTORS offers on behalf of companies, which are aiming to enter the medical device business, to achieve a substantial cost reduction and the speeding up of a series of processes by executing all the necessary processes ranging from the preparation of approval and the certification of medical devices to the supply of these to the market. Because the DOCTORS Group leverages its know-how about licenses and approvals for marketing and manufacturing, as well as various applications, companies aiming to obtain approval and certification of medical devices can entrust the Group with the necessary personnel and systems. Thus, companies can aim to obtain such approvals and certifications by bearing simple and systematic costs only.
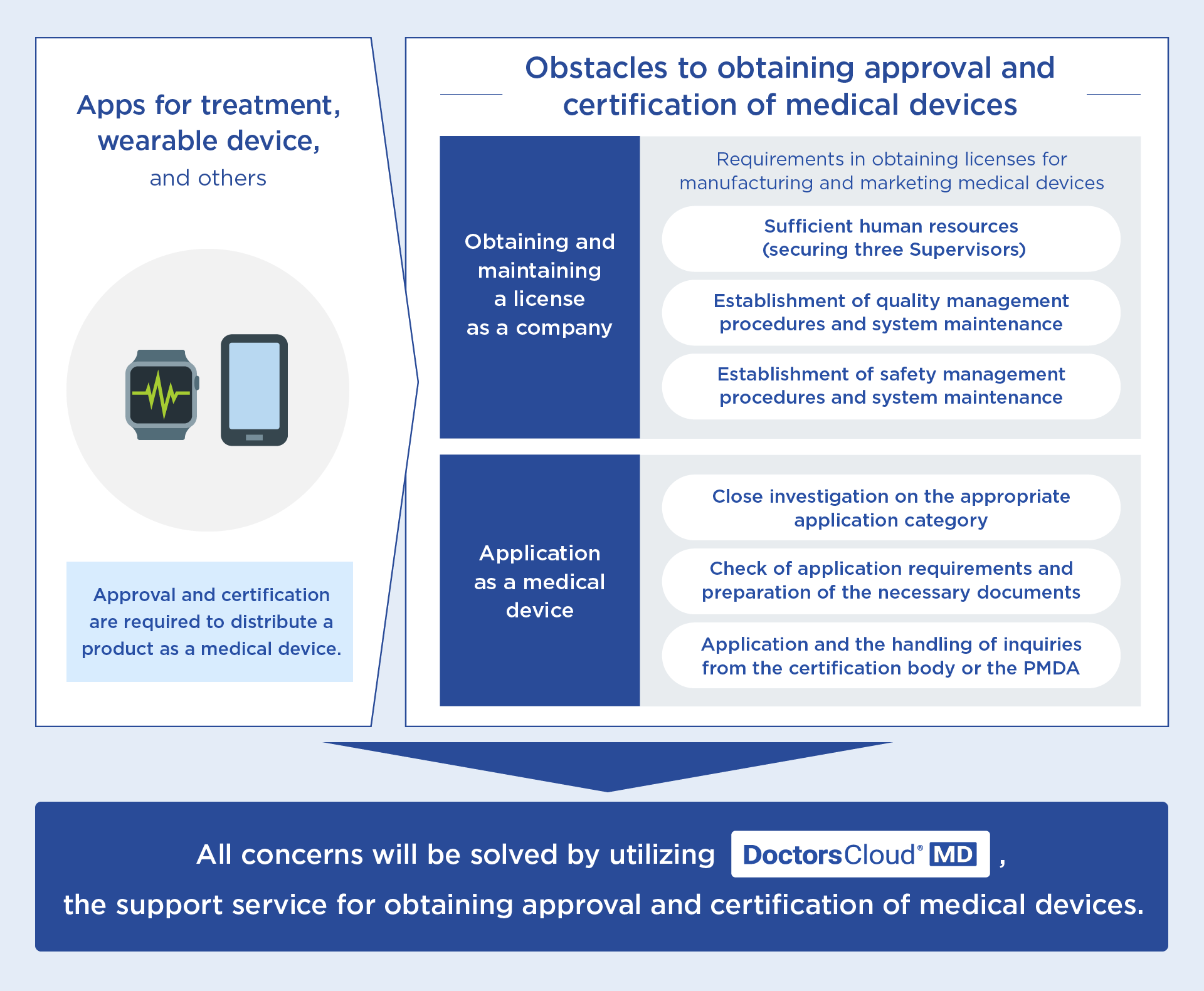
Figure: Service overview of Doctors Cloud® MD
Many startups and major companies engaged in digital healthcare services have emerged, and the development of various apps for treatment and cloud-enabled devices are in progress. Meanwhile, it is significant to obtain approval and certification for medical devices to make full use of them in the clinical environment. The processes, however, are so complicated, and those companies are required to make a wide range of specialized preparations, including obtaining licenses and approvals for marketing and manufacturing, and the assignment of personnel called three Supervisors, which are the conditions required for such licenses and approvals. Thus, companies have had challenging obstacles as a huge amount of expenses and a long period for preparation are required for them to newly enter the market of digital healthcare services to launch their products and services as medical devices.
Farmax Medical Co., Ltd., (head office: Kayano City, Nagano; representative: Chiaki Abe, hereinafter referred to as “Farmax Medical”), a group company of DOCTORS, is a professional in obtaining the approval and certification of medical devices with expertise in all processes for medical devices from Class I to IV and software as a medical device. In addition, Farmax Medical is registered as a manufacturer of medical devices and has a license and approval for marketing to supply medical devices to the market. Not only the leveraging of these licenses and knowledge but also the close cooperation between DOCTORS and Farmax Medical has made it possible to provide a one-stop support service, adapted in the digital healthcare era, for obtaining the approval and certification of medical devices.
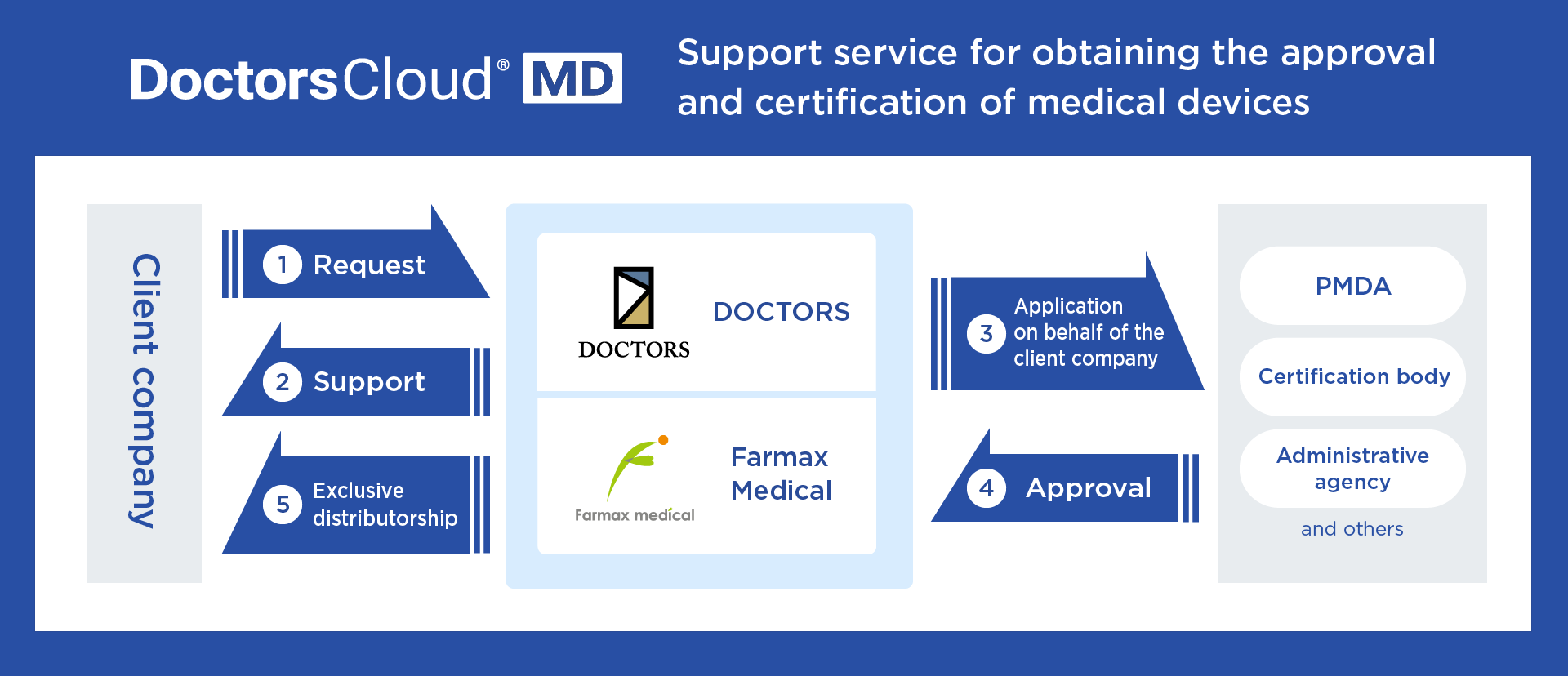
Figure: Providing support services for obtaining the approval and certification of medical devices by DOCTORS and Farmax Medical
Moreover, DOCTORS comprehensively provides services that will be required before and after obtaining the approval and certification of medical devices from strategic support for digital healthcare services to the construction of those apps and systems, to the development for distribution system to medical institutions, and to the development of telemedicine services to patients. These services can help companies, which aim for obtaining the approval and certification of medical devices, look at an exit strategy, and promote their new medical DX service business accordingly.
The DOCTORS Group will continue to provide platform services related to various medical DX for its client companies that are taking on the challenges of digital healthcare services and will accelerate further medical DX throughout the entire society through the realization of its client companies’ new services in the medical and healthcare fields.
■ Inquiry about introducing Doctors Cloud® MD, the support service for obtaining the approval and certification of medical devices
Please feel free to contact the following for an inquiry about this service as well as a request for an explanation of the service details.
[Inquiry about the use of Doctors Cloud® MD service]
Doctors Cloud Division, DOCTORS Co., Ltd.
TEL: +81-3-6263-8871 Email: doctors-cloud@doctors-inc.jp
■ Company overview
[Doctors Co., Ltd.]
Company name: Doctors Co., Ltd.
Representative: Takao Yanagawa, President & CEO
Head office: PMO Hamamatsucho II 5F, 2-3-6 Shibakoen, Minato-ku, Tokyo
Establishment: September 2016
Launch of business: October 2019
Business description: By making use of the network of active expert doctors®* based on its own guidelines, Doctors Co., Ltd., provides services that include Doctors Cloud® that offers planning and development support for commercialization of digital healthcare services, Doctors Next® that offers comprehensive support services for medical DX and digital healthcare, and Doctors Station® that offers online medical support services in collaboration with medical care.
*Expert doctors®: A network of doctors with top-class clinical experience and achievements as medical doctors, as well as a positive attitude toward digital healthcare and medical DX. More than 700 doctors, mostly specialists, participate in the network.
Website: https://doctors-inc.jp
[Farmax Medical Co., Ltd.]
Company name: Farmax Medical Co., Ltd.
Representative: Chiaki Abe, President
Head office: 8493-1 Tamagawa, Kayano-shi, Nagano
Establishment: February 2012
Business description: Providing one-stop services ranging from the designing and manufacturing to the document application for medical devices, including highly controlled medical devices (Class III and IV as well).
Website: https://farmax-med.com/
■ Inquiries from the press
Public Relations Officer, Doctors Co., Ltd.
TEL: +81-3-6263-8871 Email: info@doctors-inc.jp
*The description of Japan’s First: according to our research (as of July 2023), and as a support service for obtaining the approval and certification of medical devices in the digital healthcare field.